
|
Developed by 

|
Supported by 

|
MK-8591D (Islatravir and Lenacapavir)
Developer(s)

|
Merck Originator
https://www.merck.com/
United States Merck & Co., Inc. is an American multinational pharmaceutical company known as Merck Sharp & Drone (MSD) in territories outside of the USA and Canada. Merck was originally established in 1891, and is headquartered in Rahway, New Jersey. The company is particularly well known for developing and manufacturing biologic therapies, vaccines, medicines and animal health products. |

|
Gilead Sciences Originator
https://www.gilead.com/
United States Gilead Sciences, Inc. is a multinational biopharmaceutical company that develops and manufactures innovative medicines for life-threatening diseases, including anti-viral therapeutics for HIV/AIDS, Hepatitis B, Hepatitis C and Covid-19. Headquartered in Foster City, California, Gilead was originally founded in 1987 and is currently listed on both the S&P 500 and the NASDAQ Biotechnology Index. |
Drug structure
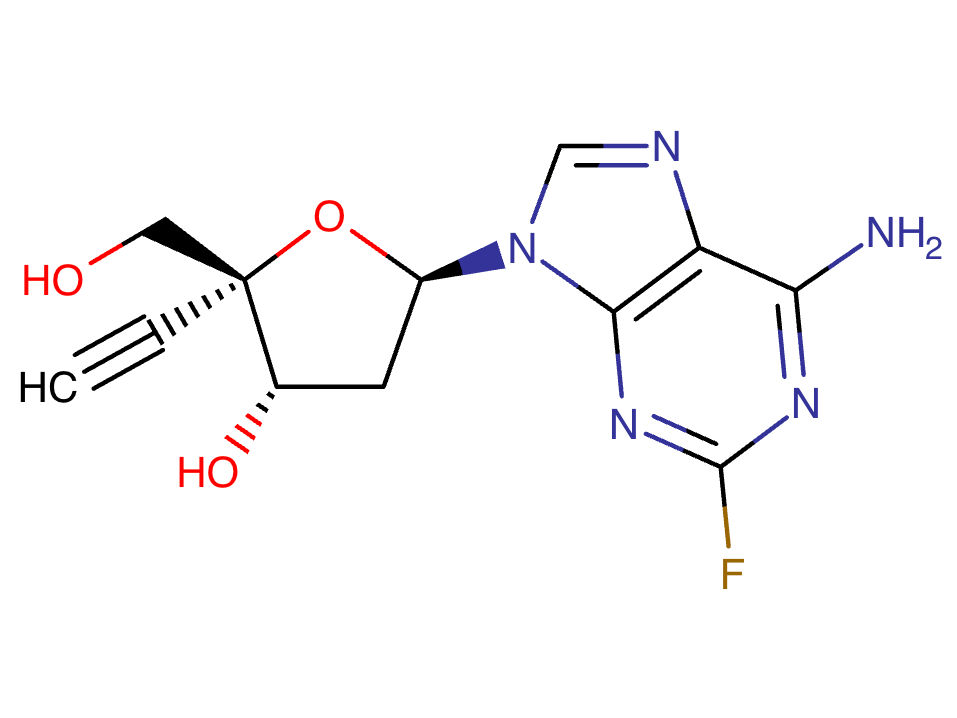
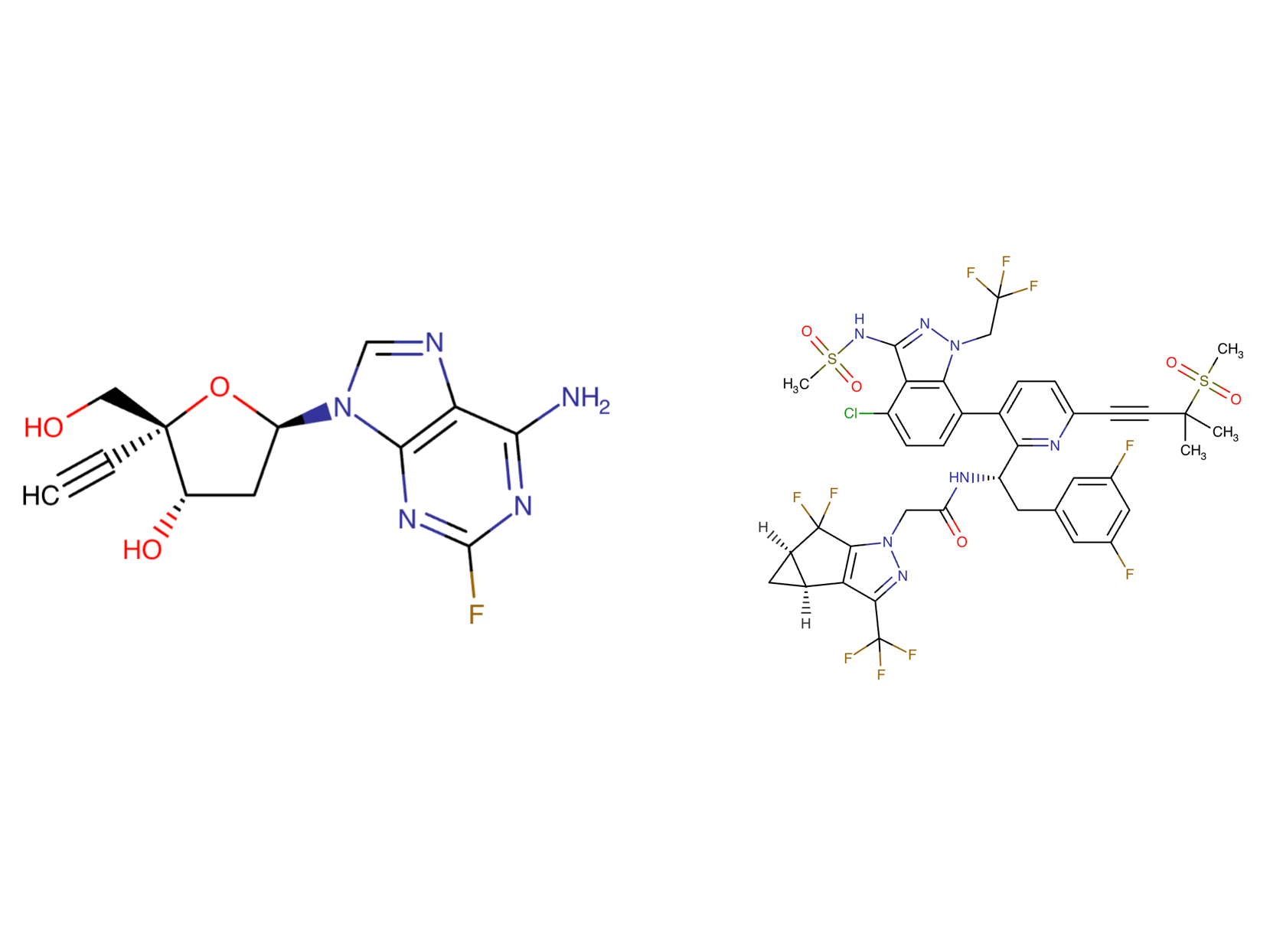
Islatravir Chemical Structure
Sourced from DrugBank

Lenacapavir Chemical Structure
Sourced from DrugBank

Islatravir and Lenacapavir Chemical Structure
Composite Adapated from DrugBank
Drug information
Associated long-acting platforms
Oral solid form
Administration route
Oral
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
Frequency of administration
Not provided
User acceptance
Not provided
Dosage
Available dose and strength
fixed dose combination of 300 mg lenacapavir + 2 mg islatravir
Maximum dose
Not provided
Recommended dosing regimen
investigational doses used: https://clinicaltrials.gov/study/NCT06630286
Additional comments
Not provided
Dosage link(s)
Not provided